is nh3 polar or nonpolar
We can summarize that polarity of a molecule is dependent on the difference in electronegativity between the atoms in a molecule. Is NH3 Polar or Nonpolar.
 |
| Is Nh3 Polar Or Nonpolar Ammonia Youtube |
Is methylamine polar or nonpolar.
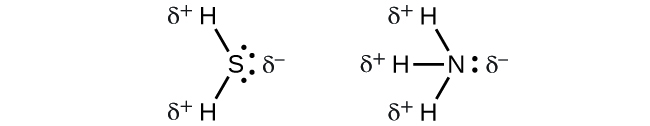
. Polarity is the result of significant electronegativity difference between atoms. In what ways would the microscope contribute to the. Yes NH3 Ammonia is indeed a polar molecule. Bromine mono chloride is also non polarBromine has electro negativity value 28 and chlorine has 30 so the electro negativity difference is 02 So that is non polar molecule.
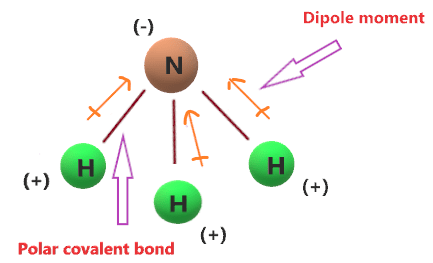
A dipole moment is given by the product of the magnitude of the charges. NH3 is a polar covalent compound because the. Ammonia 47946 views Aug 5 2013 If you look at the Lewis structure for NH3 we can see that it is not a symmetrical molecule. Is it polar or nonpolar.
According to question nh3 polar or nonpolar the answer of this question is Yes ammonia nh3 is polar molecules. The first reason is NH3 molecules is in asymmetrical. The NH3 is a polar molecule due to the large electronegativity difference between nitrogen and hydrogenThe electronegativity of hydrogen is 22 and nitrogen is 304. Ammonia NH3 is a polar molecule.
Polarity of a molecule is determined by its dipole moment. This means that NH3 has a trigonal. Is NH3 an ionic polar or nonpolar compound. H20 NH3 CCl4 CO2.
Nitrogen N is more electronegative than hydrogen H therefore charges over the nitrogen and hydrogen atoms. Yes we can say that NH3 is a polar covalent bond. The large difference in the electronegativity. Science 28102019 1829 meteor13.
A bond between two atoms or more atoms is non-polar if the atoms have the same electronegativity or a difference in. The N is the negative end the middle of the Hs represents the positive end and the dipoles cancel out. What we really need to do is look at the molecular geometry for NH3 to see if its polar or nonpolar. The answer is yes.
Conclusions Ammonia NH3 is a polar molecule and it. So Is NH3 a polar covalent or nonpolar covalent compound. When two atoms form chemical bonds they become asymmetrical molecules with polar bonds. Because methane is a non-polar molecule it is not capable of hydrogen bonding or dipole-dipole intermolecular forces.
Is NH3 polar or non-polar. You can get the difference between the N-H bond and the NH3. Is nh3 polar or nonpolar. Nitrogen forms a covalent bond with three atoms to form a molecule.
So heres the shape of the NH3 molecule--the Hydrogens right here and the Nitrogen. NH3 Polar or NonPolar. The answer to the question Is NH3 Polar or Nonpolaris that NH3 is a polar molecule because it has three nitrogen-hydrogen bond dipoles that do not cancel out. Polar molecule Methyl amine ie CH3NH2 is a polar molecule.
2 Get Iba pang mga katanungan. Unlike polar bonds non-polar bonds share electrons equally. The Ammonia molecule is polar. So is NH3 polar.
NH3 or Ammonia is a POLAR molecule because the Nitrogen N present in the molecule is more electronegative which causes the partial positive ẟ and partial negative ẟ.
 |
| Is So3 Polar Or Nonpolar Polarity Of So3 |
 |
| Are H2o And Nh3 Both Polar Molecules Why Quora |
 |
| Is Nh3 Ionic Or Covalent Or Both Ionic Vs Covalent Bond In Ammonia |
 |
| Is Nh3 Polar Or Nonpolar Nh3 Intermolecular Forces Nh3 Charge Nh3 Bond Angle Detailed Facts Chemwhite Com |
 |
| 4 12 Shapes And Properties Polar And Nonpolar Molecules Chemistry Libretexts |
Posting Komentar untuk "is nh3 polar or nonpolar"